Abstract
Background: Conflicting data exists on whether T-cell dose of allogeneic stem cell product influences transplant outcome.
Methods: Using CIBMTR database, we identified 2,736 adult patients who underwent first allogeneic HCT for AML/ALL/MDS (between 2008-2014) with PBSC from an HLA-identical sibling donor (MSD) or 8/8-matched unrelated donor (MUD). This cohort excluded ex-vivo and in-vivo T-cell depletion transplants. Correlative analysis was done between CD3 cell dose and risk of GVHD, relapse, NRM, DFS and OS.
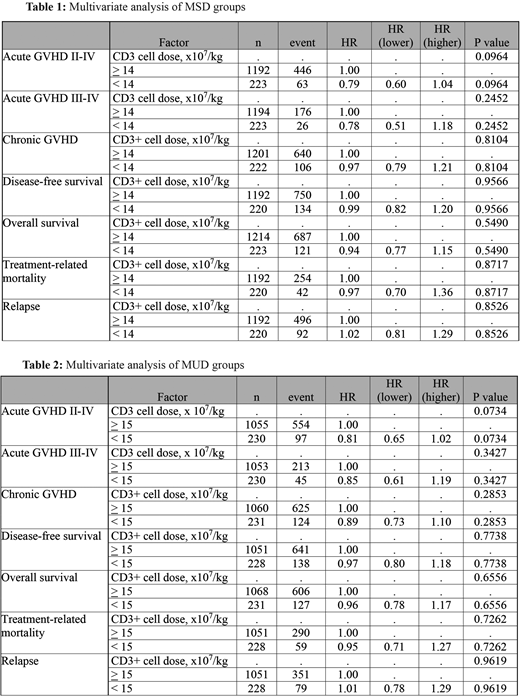
Results: T-cell dose cutoff values were identified (using maximum likelihood estimation method) to identify differential risk of aGVHD (grade II-IV) in both MSD and MUD groups. A CD3 cell dose cutoff of 14 x107 cells/kg identified MSD/low CD3 (n=223) and MSD/high CD3 (n=1214) and a dose of 15 x107 cells/kg identified MUD/low CD3 (n=197) and MUD/high CD3 (n=1102). CD3 and CD34 cell doses did not correlate. Median CD3 cell dose were 11 and 29 in the MSD/Low and MSD/High groups, respectively, and 10 and 28 in the MUD/Low and MUD/High groups, respectively. In MSD/high CD3, cumulative incidence (CI) of D100-aGVHD (GII-IV) was higher (33% vs 25%) (P value =0.009) without influence on engraftment, severe aGVHD (GIII-IV), cGVHD, NRM, relapse, DFS, or OS. In MUD/high CD3, CI of D100-aGVHD (GII-IV) was higher (50% vs 40%) (p value=0.009) and so was the CI of 6-month-cGVHD (31% vs23%) (p value = 0.02) with no influence on engraftment, severe aGVHD, 2-year-GVHD, NRM, relapse, DFS, or OS. On multivariate analysis, both MSD (table 1) and MUD (table 2) groups failed to show a correlation between CD3 cell dose and aGVHD (GII-IV) (p value =0.1 and 0.07), cGVHD (p value=0.8 and 0.3) for both groups respectviely. Sub-analysis of CD4, CD8 and CD4/CD8 ratio failed to identify cutoff values predictive of transplant outcome. Using log-rank test, the sample size was, however, suboptimal to identify difference (with 80% power at 0.05% significant level) at these cutoff cell dose.
Conclusion: In this relatively small registry study, the CD3 cell dose in the PBSCT product did not influence the risk of acute or chronic GVHD or other transplant outcome when using matched sibling or 8/8 unrelated donors.
Saad:Actinium: Consultancy. Lamb:Incysus Therapeutics Inc.: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal